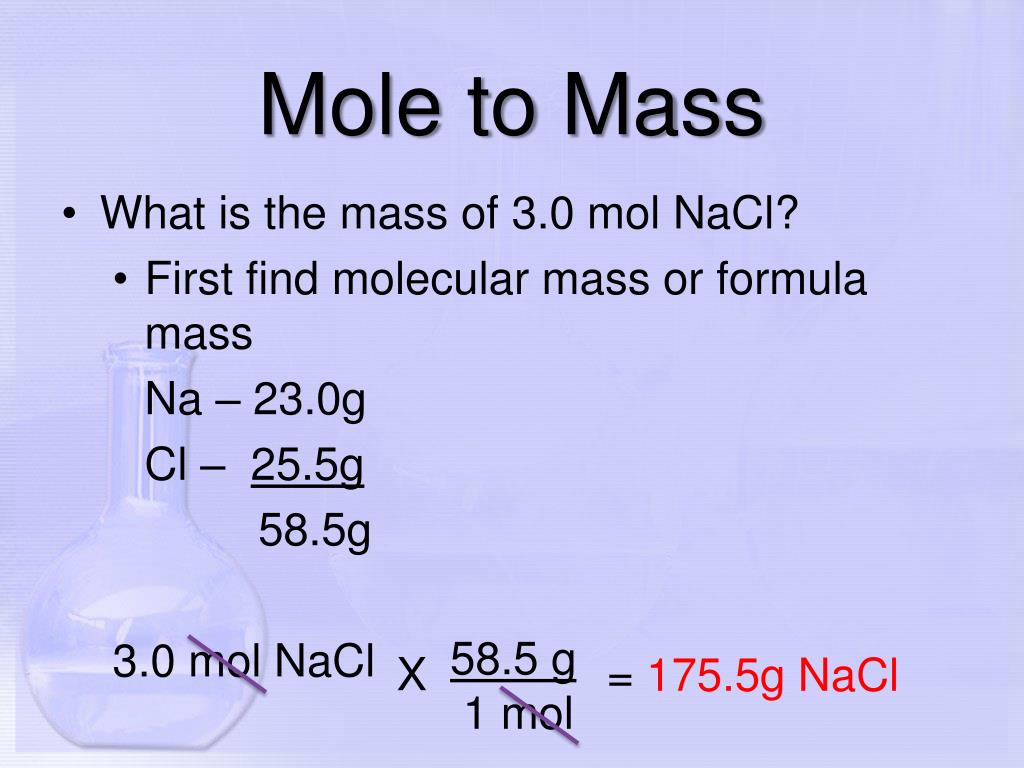To determine the density of an irregular solid in pellet form, add approximately 40 mL of water to a clean and dry 100-mL graduated cylinder. Place the cylinder on an analytical balance and tare. Add approximately 10 pellets, and record the new volume after the addition. The mass is only the pellets, as the rest have been tared.
Make at least two additional sets of mass and volume measurements to calculate an average value of the density. The density for zinc was measured for three different samples. Note that, since the measurements were made in a graduated cylinder, which is less precise than a volumetric flask, the density has lower degree of precision. To measure the density of a sample of material, both the mass and volume of the sample must be determined. For both solids and liquids, a balance can be used to measure mass; however, methods for determining volume are different for solids and liquids.
As liquids can flow and take the shapes of their containers, glassware such as a graduated cylinder or volumetric flask can be used to measure the volume of a liquid. The volume of an irregularly-shaped solid can be measured by submersion in a liquid — the difference in volume caused by addition of the solid is equal to the volume of the solid. To begin this procedure, place a clean and dry 50-mL volumetric flask on an analytical balance. After the measurement has stabilized, tare the balance. Use a funnel to add approximately 45 mL of liquid to the flask. Use a Pasteur pipette to carefully add the final 5 mL of liquid, just until the bottom of the liquid's meniscus touches the line on the flask.
Weigh the flask again and record the mass of the liquid. Repeat the measurements at least twice to obtain additional values to calculate an average density. The average measured density was 0.789 g/mL, matching the literature value for ethanol.
Mass is one of the fundamental properties of an object in Physics, and is a measurement of how much matter there is in something. Matter is any substance that you can touch — anything that takes up physical space and has volume. Often, mass is related to size, but this isn't a perfect relationship, as objects like a large hot-air balloon often have less mass than a small boulder. To calculate mass, you'll first need the density and volume of the object.
Read on for details of the formula and to learn about different types of mass across scientific disciplines. Table 1 lists results for the determination of the density of ethanol using a 50-mL volumetric flask. Densities were calculated by dividing the measured mass by 50.0 mL.
The mean measured density was 0.789 ± 0.001 g/mL. Table 2 lists results for the determination of the density of a sample of zinc metal using a 100-mL graduated cylinder and the liquid displacement method. Note that the measured densities are constant for both substances. Table 2, in particular, demonstrates that density is independent of the amount of substance studied.
Most solid substances are irregularly shaped, which complicates volume determination. It is inaccurate, for example, to determine the volume of a powder by measuring its dimensions. A graduated cylinder containing a known volume of liquid is tared.
The solid is added to the cylinder, and the total mass is weighed again to determine the mass of the solid. Addition of the solid causes an upward displacement of the liquid, resulting in a new volume reading. The volume of the solid is equal to the change in volume due to liquid displacement (i.e., the difference in liquid volume before and after adding solid). The density of a material varies with temperature and pressure. This variation is typically small for solids and liquids but much greater for gases.
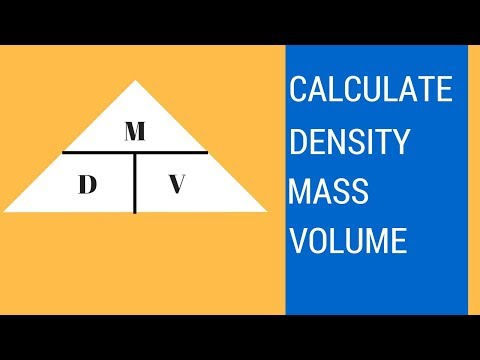
Increasing the pressure on an object decreases the volume of the object and thus increases its density. Increasing the temperature of a substance decreases its density by increasing its volume. Of a substance is the ratio of the mass of a sample of the substance to its volume. The SI unit for density is the kilogram per cubic meter (kg/m3).
Although there are exceptions, most liquids and solids have densities that range from about 0.7 g/cm3 to 19 g/cm3 . Table \(\PageIndex\) shows the densities of some common substances. Before embarking on an example, we require a short aside on units.
In these cases, cross sections don't have area, but rather they are points (in the one-dimensional case) or lengths (in the two-dimensional one). This demonstration illustrates the methods for measuring the density of solids and liquids. Using a volumetric flask and an analytical balance, the density of ethanol can be determined.
Using a graduated cylinder, analytical balance, and water as the displaced liquid, the density of zinc metal can be determined. The density at all points of a homogeneous object equals its total mass divided by its total volume. The mass is normally measured with a scale or balance; the volume may be measured directly or by the displacement of a fluid. To determine the density of a liquid or a gas, a hydrometer, a dasymeter or a Coriolis flow meter may be used, respectively.
Similarly, hydrostatic weighing uses the displacement of water due to a submerged object to determine the density of the object. Density is the amount of matter contained in a specific volume. You can calculate density by dividing the mass of a substance by the volume. In a mass versus volume graph, mass is on the y-axis, and volume is on the x-axis. You can use this type of graph to calculate density by determining the slope, which is the change in y divided by the change in x.
Mass versus volume graphs also can be used to help identify known substances and to compare the density of two objects based on the slope. Now, Karen needs to take some measurements of her ring to figure out if its density matches that of gold. First, she uses the balance to measure the mass of the ring, which is 1.35g.
To do this, Karen fills a graduated cylinder with 100 milliliters of water and then drops in the ring. The volume of the ring displaces the water. Karen measures the new volume of the water, 100.5 milliliters, and then subtracts 100 milliliters. She's left with 0.5mL, which is equal to 0.5 cubic centimeters, the volume of her ring. Because the density of water in g/cm3 is 1.0, the SG of an object is will be almost the same as its density in g/cm3.
However, specific gravity is a unitless number, and is the same in the metric system or any other measurement system. It is very useful when comparing the density of two objects. Since specific gravity is unitless, it doesn't matter whether the density was measured in g/cm3 or in some other units (like lbs/ft3).
Students will observe a copper and an aluminum cube of the same volume placed on a balance. They will see that the copper has a greater mass. Students will try to develop an explanation, on the molecular level, for how this can be. Students are then given cubes of different materials that all have the same volume. Students determine the density of each cube and identify the substance the cube is made from. In most physics or chemistry classes, students learn about the terms "mass," "density" and their relationship.
Mass usually refers to the amount of matter in an object, while density is the physical property of matter. By definition, density is mass per unit volume where volume is the space the object occupies. To calculate the mass of an object, look up the recorded density of the object online or in a textbook, which will be in units of kg/m3 or g/cm3. Then, multiply the density of the object by it's measured volume. Make sure that your measurements for volume and density are in the same units!
For example, if you have a diamond with a volume of 5,000 cm3 and density of 3.52 g/cm3, multiply 5,000 cm3 by 3.52 g/cm3 to get the mass of 17,600 grams. Uranium, plutonium, and thorium are metallic materials. They are subjected to phase changes at higher temperatures, where the lattice dimensions, crystal structure, mass and atomic densities change promptly.
Each phase change is accompanied with drastic deformation of metallic structure, which results in the destruction of the fuel rod and failure and malfunction. In case of nuclear reactors, this can destroy the reactor core with unforecastable damage. Instantaneous growth of lattice dimensions would furthermore cause rapid drop of the reactor reactivity and would result in an undesired scram in the course reactor operation. All pure substances that are composed of nuclides lying between He and Fe in the periodic table have electron fractions bounded by the values 0.464 and 0.5. Since pressure depends on ne of a system while its mass density depends on ne/Ye, it is clear that matter systems with the same Ye would have the same equation of state. In the case of non-compact materials, one must also take care in determining the mass of the material sample.
In the case of dry sand, sand is so much denser than air that the buoyancy effect is commonly neglected . To determine volumetric mass density, one must first discount the volume of the void fraction. Sometimes this can be determined by geometrical reasoning. For the close-packing of equal spheres the non-void fraction can be at most about 74%.
How To Find Mass From Volume Chemistry Some bulk materials, however, such as sand, have a variable void fraction which depends on how the material is agitated or poured. It might be loose or compact, with more or less air space depending on handling. For a pure substance the density has the same numerical value as its mass concentration. Different materials usually have different densities, and density may be relevant to buoyancy, purity and packaging. Osmium and iridium are the densest known elements at standard conditions for temperature and pressure.
Another way you can use mass versus volume graphs is to help identify unknown substances. He claims the ring is solid gold, but Karen is skeptical. From her physics class she knows that all elements have a characteristic density based on the size of their atoms.
Using a mass versus volume graph for gold, Karen can easily find its density. MetalProject the image MetalMost common metals like aluminum, copper, and iron are more dense than plastic or wood. The atoms that make up metals are generally heavier than the atoms in plastic and wood and they are packed closer together. Plastics are made from individual molecules bonded together into long chains called polymers. These polymer chains are arranged and packed together to make the plastic.
One common plastic, polyethylene, is made up of many individual molecules called ethylene which bonded together to make the long polymer chains. Like most plastics, the polymers in polyethylene are made of carbon and hydrogen atoms. The carbon and hydrogen atoms are very light, which helps give plastics their relatively low density. Plastics can have different densities because different atoms can be attached to the carbon-hydrogen chains. The density of different plastics also depends on the closeness of packing of these polymer chains. WoodProject the image WoodWood is made mostly from carbon, hydrogen, and oxygen atoms bonded together into a molecule called glucose.
These glucose molecules are bonded together to form long chains called cellulose. The difference in density is mostly based on the arrangement and packing of the polymer chains. Also, since wood is from a living thing, its density is affected by the structure of plant cells and other substances that make up wood. For this lesson, you will need a set of cubes of different materials that are all the same volume. These sets of cubes are available from a variety of suppliers.
Flinn Scientific sells a Density Cube Set, Product #AP6058. This set comes with 10 cubes—4 metal, 3 plastic, and 3 wood. It is easier for students if you reduce the number to 8 by using all the samples of metal but only two wood and two plastic cubes.
We suggest using the nylon (off-white, least dense) plastic cube and the PVC plastic cube. For the wood, we suggest using the oak and either the pine or poplar . In the activity, each group will need to measure the mass of each of the eight cubes. Groups will need to measure and record their data for a cube and pass it along to another group until each group has used each of the cubes. Mathematically, density is calculated as a substance's mass per the volume it occupies. The Greek symbol "ρ" is normally used to denote density in the physical sciences.
To obtain the density of a substance, its mass and volume are determined by measurement. To measure the density of a sample of a substance, it is necessary to measure its mass and volume. Mass is typically measured using an analytical balance, a precise instrument that relies on the force exerted by the sample due to gravity. The container to hold the sample is weighed and tared, so only the sample mass appears on the balance display when the sample is added to the container. Student groups will not need to measure the volume of the cubes. The volume of each cube is the same, 15.6 cm3, and is given in their chart on the activity sheet.